-
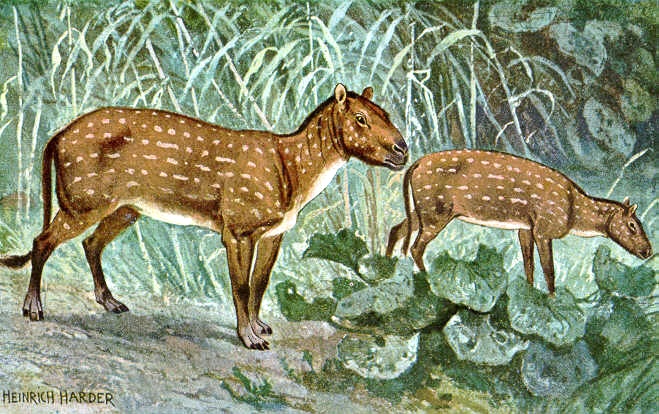
Archaeopteryx

A a detailed description of the creature, and an answer to the question of why it is regarded as important evidence for evolution.
Archaeopteryx is the name given to a creature from the Late Jurassic Period (150 million years ago). The characteristics of this animal are strange to the modern eye, as it has a similar body shape to a European Magpie, but has many features in common with Mesozoic dinosaurs, such as the sharp-toothed jaws, clawed fingers, and a long bony tail. This bizarre phenotype has led the archaeopteryx to become a popular example of a transitional fossil, which is closely related to the origin of modern day birds.
Animal Info
The archaeopteryx lived in what is now part of Germany, in an era during which Europe was far closer to the equator, and therefore had a far more tropical climate. The creature would have been 50cm long, and weighed around 0.9 kilograms. It had a lightweight, feathered body, a flat sternum, large eyes, conical teeth (1), a long neck, and long thighs supported by short calves. Physical data suggests that the creature was capable of flight by this stage, but the ability was far from refined. Despite this, the archaeopteryx’s well developed flight feathers are widely considered the most important part of the fossils, especially in terms of using them as evolutionary evidence, as they have a very similar structure to that of modern birds.
These proto-birds developed over a far longer period than their modern avian counterparts. It is estimated that after hatching, archaeopteryxes took around 32 months to grow to their adult size (all known specimens died and were fossilised as juveniles). They could potentially have lived for up to forty years (2), compared to just twenty years for a contemporary European Magpie (3).
Evolutionary Status
The first archaeopteryx fossil was discovered in 1860, by a German palaeontologist named Christian Erich Hermann von Meyer. The fossil consisted of a single feather, buried in the Solnhofen Limestone of South Germany. The fossil is widely regarded as the first evidence of the archaeopteryx, but little info could be extrapolated from a mere feather, and there is even some uncertainty that the creature it belonged to was even a member of the same species.
In later years, there have been several subsequent discoveries, all far more useful. Detailed analysis has provided strong evidence that the archaeopteryx shows the intermediate evolutionary stage between the Jurassic dinosaurs and modern day birds.
Around the time of their discovery, however, the authenticity of the archaeopteryx fossils was repeatedly questioned. Even as late as 1985, papers were published by physicist Lee Spetner and astronomer Fred Hoyle which set out to prove that the Berlin and London specimens were simply forgeries (4).
The controversy continues to this day, mainly consisting of the anti-evolution arguments perpetuated by religious fundamentalists, but by and large the archaeopteryx fossils have been accepted as solid evidence for the theory of evolution. These fossils are very important to biologists and archaeologists alike because they lay out the ancestral origins of modern-day birds as well as providing a vital clue as to the evolutionary lineage between the organisms of the Jurassic Period and those of the present day. This key position in scientific history has led the archaeopteryx specimens to sit among the most well-known fossils of all time.
- Confirming that they were carnivores.
- This is only a vague estimate, as I have found little in the way of precise figures.
- Both cases assume that the organism lives out its full potential lifespan. Obviously, most would die of unnatural causes long before.
- Though the reasons given, such as how the slabs of rock around the fossil split so smoothly, merely indicated that they were unfamiliar with lithification (sediments compacting under pressure to become solid rock).
-
Austrian Archduke Assassinated

 A newspaper article about the assassination of Franz Ferdinand in 1914, written from a contemporary perspective.
A newspaper article about the assassination of Franz Ferdinand in 1914, written from a contemporary perspective.People in the Austro-Hungarian town of Sarajevo were shocked to see archduke Franz Ferdinand gunned down by a gang of Bosnian terrorists.
On June 28th, 1914, Archduke Franz Ferdinand of Austria was travelling through Sarajevo. He had been inspecting Austrian troops nearby, and was heading to open a new state museum in the town. For the Archduke it was a particularly special occasion, as it was one of few events in which his wife, Sophie, was allowed to ride beside him. In what was in retrospect a great failure of judgment, Ferdinand demanded that the motorcar be open-topped, and that there be as little security as possible, so as not to appear too militaristic. These goodwill gestures made the Archduke an easy target for his killers, a group of state hired terrorists known colloquially as the Black Hand Gang. Seven members were lined along the streets of Sarajevo armed with pistols and bombs. Only two, however, actually took action: Nedeljko Čabrinović, and Gavrilo Princip. Both are in their late teens, and both have been reported to be dying of tuberculosis. At 10:10am, Čabrinović tried to kill the archduke with a small bomb, but it bounced out of the automobile and destroyed the car behind, injuring 20 civilians. One local pedestrian told our reporters:
“It was a young man of maybe twenty. He bashed a little black object against a lamp post, and hurled it at the car. The car sped up a little, and the thing bounced off the hood at the back. It exploded behind, and the young man took a swig of something, and then jumped into the river. He was obviously trying to kill himself but the river was too shallow, and he got dragged out by the crowd, throwing up everywhere.”
After the failed bombing, the other assassins (yet to be identified) failed to act as the remains of the motorcade passed by. The Archduke went on with the day’s duties, but insisted on visiting his wounded colleagues in hospital. Ferdinand’s driver was not informed about the change in plan, and so took a wrong turning, ending up outside Schiller’s delicatessen on Franz Joseph Street, where Princip had been buying a sandwich. The young assassin fired two bullets into the car, fatally wounding both Franz and Sophie. It is a great shame that such a terrible crime could have been so easily prevented. After shooting the couple, Princip attempted suicide, but his gun was wrestled off him, and the cyanide once again failed.
Police are now attempting to trace the remaining assassins, as the empire contemplates what it will do now that such an important member has been lost. Already, political tensions are starting to rise, and the outbreak of war may come soon.
-
Evolution of the Horse

An explanation as to how changes in habitat over millions of years may have caused the modern horse to evolve from an animal no bigger than a fox.
Among the earliest known ancestors of the modern horse (Equus) is Hyracotherium Eohippus, which is believed to have lived around 55 million years ago. The creature was around 60 cm long and weighed 23 kg with four-toed feet and a slender body rather unlike what we would now call a horse. Hyperaesthesia lived in the woodlands of North America and Western Europe during the Eocene era, the hottest period in pre-history. During this time, the ground was mostly soft and moist (particularly in the forest areas), and so the animals were best served by walking with their toes spread out, and their body weight spread evenly between them. This allowed them to walk along soft surfaces without sinking in and becoming stuck.
Eventually the main land became harder and dryer, meaning that the original configuration was no longer necessary (and indeed potentially detrimental). With the ground more stable, the mammals could now run at a much faster pace, so the evolutionary emphasis shifted from buoyancy to power. In order to achieve this, the horses became larger, with their limbs lengthening, and their toes becoming more pointed. With the later generations of the horse now pushing off from one toe, their land speed was dramatically increased, allowing them to more easily outrun predators. There have been other changes, too: with each generation the originally wide-spread toes moved closer together, eventually becoming the now familiar hoof. Meanwhile the teeth changed shape and became more numerous (particularly molars) to keep up with changes in diet.
By the time Homo Sapiens Sapiens developed, the horse had grown to a height of 1.6 metres, and has earned an unofficial title as “Man’s second-best friend” due to their superb pulling and carrying ability making them a vital tool in the formation and development of human civilisation.
-
DNA

A brief summary of what DNA is, with details about its function and discovery.
Deoxyribonucleic acid is a substance which forms the genetic structure of all living organism. DNA is found in the chromosomes contained within the nuclei of living cells, where it is arranged in a double helix structure. The structure is made up of small sections called genes. Each gene helps to control the production of proteins within a cell, and through this they determine the physical characteristics of the organism. The individual strands of DNA are made up of combinations of six different chemicals: Adenine, Thymine, Cytosine, Deoxyribose sugar, Guanine and Phosphate. The chemicals are arranged in nucleotides, with a phosphate molecule first, then a sugar molecule, and then the “base” (adenine, thymine, cytosine, guanine). A DNA strand is formed when two nucleotides lock together. Cytosine is always opposite a guanine nucleotide, because the two molecules are shaped to perfectly lock together, making them “base pairs”. Adenine and thymine are also base pairs. The base pairing rule allows DNA to be replicated when cells divide, because the strands on one helix can have their base pairs made up to match.
Genes determine the phenotype (what the resulting organism will look like). There are often different forms of the same gene (each of which has, for instance, a different eye colour), and these are known as alleles. When a new organism is formed –sexually, it will often inherit two different alleles , one from each parent, but only one allele will be used, while the other remains dormant. We call one allele “dominant” and the other “recessive”. In human eyes, for instance, the allele for brown cornea is dominant to its blue counterpart, so if a person with brown eyes (BB) mates with a person with blue eyes (bb), their child will have brown eyes (Bb). However, if a Bb person mates with a bb person, at least some of their children will have blue eyes again.
As mentioned earlier, DNA molecules are coiled into structures called chromosomes. These consist of one double-stranded DNA molecule tightly coiled around proteins called histones. The number of chromosomes in a cell is different depending on the species. Humans normally have 46, of which there are 22 pairs common to both sexes. The 23rd pair, for females, consists of two X shaped chromosomes, but males have a mismatched pair of one X and one Y shaped. It is this difference which determines a person’s gender. Other species have different numbers of chromosomes: Goats have 60, cats have 38, kangaroos have 16, and snails have 24. The number of chromosomes that an organism has is believed to depend on the number of evolutionary stages which came before its own phenotype.
Occasionally, people can be born with the wrong number of chromosomes. People with Down Syndrome, for instance, have an extra 21st chromosome, and girls with Turner Syndrome are born missing one X chromosome. These birth defects often lead to slowed development, and physical deformities.
DNA as we now know it was first discovered in 1953 by Cambridge University scientists James Watson and Francis Crick. Up until that point, it had been accepted that DNA was the substance which carried genes from one generation to the next, but little was known about what DNA actually was, or how it worked.
Watson and Crick used the research done by Rosalind Franklin, in which she used X-ray diffraction to take pictures of crystalline DNA. Watson and Crick used this research to work out the double helix structure of DNA to match the photographs. Their proposal is now accepted by scientists worldwide. Watson and Crick were awarded the Nobel Prize in 1962, but by then Franklin had died of cancer, and the prize is not allowed to be awarded posthumously.
-
Titanic Narrative


A short story about one or more passengers on the doomed maiden voyage of the RMS Titanic.
The cold water quietly washed along the huge metal hull as the enormous ship sat in the dock to await the coming passengers. All around the harbour there was a gigantic swarm of people rushing towards the water. In amongst the bustling crowd was a middle-aged couple involved in what had been the fifth such argument of that day.
As the couple moved forwards, their bickering became louder and louder until finally they stopped to look at where they were going. There an incredible sight met their eyes: standing before them was the largest vessel they had ever seen: the vast black hull seemed to dwarf the entire county, and the great white superstructure above it seemed like a palace, with four huge funnels poking up into the heavens. As the couple gazed at the magnificent machine that stood before them, they knew there were in for a considerable treat.
Before the cruise could begin, however, there was the matter of getting on board: access, it seemed, could only be achieved via a small metal causeway from the edge of the deck to the ship’s hull. At the end of the causeway was a surly-faced guard clumsily ushering hordes of middle class people towards the ship. After standing rather impatiently behind the bustling crowd, they finally reached the guard. The guard lazily stared at them and said
“Let me see your tickets!” A large ticket was handed to him, which he then snatched up and shouted,
“Thomas and Anita Brooklands, Second Class!” He then looked back at the couple and asked the gentleman “So”, he checked the ticket, “Thomas, what brings you aboard?” Thomas, taken by surprise, gulped and replied
“M-M-M-my wife and I-I…” he trailed off, racked with shame.
“We are going to America to sell some of our wine!” interjected Anita. The guard, evidently finding this hilarious, chortled
“And which of you’s gonna do the deals? Will you do the talkin’, or ‘sit gonna be old stutter box? Heh heh heh!!” Seeing Anita’s look of stern disapproval, he cleared his throat and, with an obviously forced smile, gestured towards the ship. Thomas and Anita were all too happy to continue.
Thomas felt deeply embarrassed: his stammer had come back. When he was a small child, his father had always been ashamed of his son’s attempts to speak.
“How” he would often grumble “are you to carry on the Brooklands legacy if you cannot even manage to get a sentence out of that jittering mouth of yours?” Thanks to the constant stammer, Thomas had never been the great figure that his father had hoped for. Edward Brooklands had one of the largest vineyards in Britain, and had made enough money to buy a huge country mansion. Thomas, on the other hand, was struggling to sell a single bottle without assistance, and his family had long since lost all faith in him.
As Thomas arrived aboard, all these memories were swiftly pushed to the back of his mind by the sights which greeted him: the interior walls of the ship were as grand as those in the mansion, and the room that they were in contained the grandest staircase that either Thomas or Anita had ever seen. Indeed, Anita seemed to be struggling to take in all of the grandeur; she stood in the centre of the floor, quietly pivoting on one foot and spinning around to look at every nook and cranny in turn. Thomas was now rather glad that this was only the Second Class area, as anything more luxurious might have caused her to faint. Clearly, this was going to be an interesting voyage.
*
Four days had now passed since boarding, and Thomas was finally starting to enjoy the trip. After making such a scene in the entrance hall, Anita had finally settled down and accompanied Thomas to explore the ship’s luxuries. They had loved the dining hall in particular, as it easily put the best restaurants to shame. Afterwards, Anita would always bury herself in the library for hours, which somewhat irritated Thomas as the smoke room was always full of drunken men smoking several cigars at a time, and nearly setting the wood panels on fire! Best of all, though, was that the stammer had all but disappeared, allowing him to negotiate a deal for three cases of his father’s wine with another eccentric gentleman over dinner. It didn’t seem that anything could possibly go wrong…
“Thomas! Thomas! Oh Tommy, wake up!” cried the shrill voice from beyond Thomas’ dreams. At last, after much shaking of the bed, Anita got his attention.
“W-W-W What’s happening?” he asked, sitting bolt upright on the bed “Is everything alright? You haven’t seen another black cat have you?” Anita looked terrified. All around him, Thomas could hear confused murmurings and anxious gasps. Anita wailed
“Thomas, come quick: I think we’ve hit something!” Thomas quickly got dressed and ran out of the door. When they reached the promenade, it all became clear: there was ice all over the deck, and the ship was leaning slightly to one side. Thomas slowly turned to Anita and stuttered
“I-I-I T-T-Think w-we’re sinking!” At that moment, Anita gave a scream of terror that made everyone on the deck hold their ears. Then she shouted
“We’re sinking! WE’RE SINKING!!” and all around her the other passengers started to panic and rush around. Thomas deeply wished he had been wrong.
For a long time there was silence from the crew, and the passengers were not as jittery (granted, the ship still felt relatively calm given that it had supposedly rammed an iceberg), but Anita was still insistent that they should get ready to leave as soon as possible. She and Thomas were in their cabin, meticulously packing away everything they had brought with them. It was then that they heard someone shout.
“They’re lowering the lifeboats!” Anita jumped and said
“Quickly, let’s get off this wretched ship!” and Thomas was only too happy to agree.
Getting there, however, was easier said than done, because there seemed to be no way of accessing the lifeboats from their current position. At last, after much desperate searching, they found a way in, but it was guarded by a large thug with a red face and a menacing frown.
“And just where do you think you’re going?!” he roared at the couple “This area is First-Class only!” Thomas saw that any reasoned argument would be impossible and that anyway he had no time, so he dug deep into his coat pockets and pulled out as many coins as he could find. He looked back at the guard and held them above his head. The guard looked blankly at them for a moment, then replied “Works for me!” and stepped aside. Tossing the coins in the air, Thomas sped across the deck, with Anita breathlessly following. Thomas saw her glare at him.
“What did I tell you?” she piped “I knew we should have gotten First Class tickets!”
When they finally reached the boat deck, the commotion was unbelievable: the First Class passengers were all pushing and shoving to get away, while gormless crewmen tried in vain to hold them back with cries of “Women and children only; NO MEN!”. Many of the wealthy lords were helping their less-than-grateful wives into the enormous wooden boats. Thomas and Anita tried to blend in with the crowd as they headed for the next boat. Anita was losing her nerve. “Oh, Thomas, whatever will happen to me?” she wailed.
“You’ll be perfectly fine.” replied Thomas, as the lifeboat was moved into position.
“Right,” called one of the crewmen, “In ya come!” As the front row of the bustling crowd moved forwards, Thomas led Anita to where the guards were ushering them. Trying to seem helpful, Thomas picked Anita up by her waist and tried to lift her across the edge of the deck. Then there was trouble: As the passengers hurried to evacuate, one of them violently knocked the davvy holding the lifeboat up, and the craft swung forward, leaving Anita directly above the icy water. She started to panic, screaming and wriggling as Thomas frantically tried to pull her back on board. Just as Thomas thought he had regained his grip, disaster struck: the passengers, shocked at how unstable the lifeboat seemed to be, were in a frenzy of panic, and were clumsily rushing around the deck. As Thomas was lifting Anita up, a rather heavy man came thundering past him and crashed, hard, against Thomas’ back. Thomas began to lose his balance and ended up leaning forward. Anita then seemed to become heavier and heavier before finally she slid out of his hands altogether. Thomas watched, aghast, as she plunged, without even a faint whisper, into the water and then disappeared into the deep blackness. Thomas began to look around helplessly, trying to come to terms with what had just happened
“A-A-A-A-Anita! N-N-Noooo!!”. He caught the eye of another passenger and said “M-m-m-my-” but words now completely failed him. He looked glumly at the ocean, and saw only a black nothingness.
-
Neon

An information sheet about the noble gas.
Atomic Info
Neon (Ne), is the second lightest of the noble gases, with an atomic mass of 20. Each neon nucleus is comprised of 10 protons and 10 neutrons, and orbited by 10 electrons (arranged in a 2, 8 configuration).
Properties
Neon remains in gaseous form even in temperatures far below zero: It melts at -248.6°C, and boils at -246.1°C. It appears as a colourless gas.
Discovery
The presence of neon was first noted in 1898 by two chemists: Sir William Ramsay of Scotland and Morris Travers of England, who extracted the gas by boiling a condensed sample of the atmosphere. It was quickly discovered that when electrified, the gas gave off a “blaze of crimson light”. The name “Neon” was originally derived from a Greek word, meaning “new one”.
Uses
Perhaps the best known use for neon is the infamous neon tube light. This is made up of a hollow tube of glass filled with gas. Electrodes are then inserted into the ends of the tube and, when a voltage is applied, electrify the gas to produce a coloured glow. Neon typically glows yellow/orange.
-
Electricity Usage in the United Kingdom


An explanation of how much is used overall, as well as where and when the peaks are.
The United Kingdom, as a fairly well developed nation, uses a great deal of electricity per capita compared to many other countries around the world. Overall the UK uses an estimated 6000 kWh per person every year, only 7% of which comes from renewable energy.
The average amount of energy used in the UK varies according to several factors: For instance, between 6 and 9AM, there is a sudden increase in the levels due to the vast numbers of people getting up at that time for the “nine-to-five routine”, and across the nation there are suddenly millions of radios, televisions, toasters and kettles coming on as the entire population gets ready for the day. There will also be huge surges in demand a few hours later as thousands of offices power up multi-million computers and telephones for the rest of the day. Other peak times include various points in a television schedule, as all of the viewers switch on the kettle at the same time. There are also times at which the demand for electricity is relatively low, such as around midnight – when most of the population are in bed – and summer time – when people spend less time inside the house.
Unanticipated surges in power demand are often not good for power stations, which tend to take a long time to get running and thus cannot handle rapid changes in power output. Electricity companies therefore have to work out when the next increase in demand will come and re-plan their schedules accordingly.
-
Light Bulbs


The history and working of filament bulbs.
A standard filament light bulb is composed of a tungsten filament suspended on two metal wires inside a glass sphere. The bulb derives electrical power from two metal contact points at the base of the bulb, which connect to the electrical circuit inside the light fitting. Electricity flows into the bulb through one of the wires until it reaches the filament. When the current reaches the resistor, electricity is converted into heat, and the filament soon heats up to 2500°C, at which point it produces a bright glow. To prevent the filament from burning, the interior of the bulb is filled with inert argon gas.
In an environmentally friendly fluorescent light bulb, there is a sealed glass tube, the inside of which is coated with phosphor and mercury. When electrons are forced through the bulb’s two electrodes, the mercury evaporates and gives of ultraviolet photons. When the UV light collides with the phosphor powder at an atomic level and the phosphor then gives off a visible white light. By using the otherwise wasted ultraviolet light, fluorescent bulbs are far more efficient than traditional filament ones.
The History of the Light Bulb
An early predecessor of the modern filament bulb appeared in 1810, when an English chemist named Humphry Davy invented a device known as the arc light. The arc light had a similar setup to the filament bulb, with two wires connecting a battery to a strip of charcoal. The charcoal would glow when given an electrical charge (surrounded by “arcs” of electricity). The next step was taken in 1840 by a physicist and chemist by the name of Joseph Swan, who designed a light bulb which used a carbonised filament in a partial vacuum. The bulb worked, but the carbonised paper used for the filament was highly temperamental, and so Swan spent thirty-five years searching for a better material before finally settling on cotton thread. His new bulb (first demonstrated in 1878) managed to shine for over thirteen hours.
Meanwhile, over in America, the famous Thomas Alva Edison had drawn up a remarkably similar invention which also used a cotton filament. Edison’s prototype beat Swan’s burning time by well over an hour. Edison then bought his rival’s patent (with Swan subsequently being largely forgotten) and set to work on further extending the bulb’s life span. In 1880, he unveiled a new bulb -this time with a bamboo fibre filament- which lasted for well over a thousand hours. This stage of the light bulb’s evolution was greatly helped by the mercury vacuum pump, invented by German scientist Herman Sprengel, which allowed the bulbs to be almost completely emptied of oxygen. The glass bulb design was borrowed from Canadian scientists Henry Woodward and Matthew Evans, whose patents he also bought.
Moving into the twentieth century, the first tungsten filament bulbs were produced by America’s General Electric Company. Later decades then saw the introduction of neon lighting, followed by photography flashbulbs’ halogen bulbs, and most recently the Energy Saving bulbs. The light bulb is now a common feature around the developed world, appearing in multiple variations throughout nearly all houses and public buildings.
-
Surface Area of Leaves and Cells

A description how a leaf and a cell are adapted to ensure that they have a large surface area in relation to their volume and explanation of why they need a large surface area, using diagrams to help.
In order to function efficiently, both a leaf and a cell require a large surface area in relation to their internal volume.
Why cells need surface area
In order for a cell to take in water and nutrients, the substances must defuse through the cell’s membranes. In order to take in a sufficient amount of nutrients, the cell membrane must be made as large as possible. In particular: the surface area must be large enough for the cell’s internal volume. A cell with a large surface area and a small internal volume will be able to take in nutrients more efficiently than one with a small surface area and a large internal volume (1).
How cells ensure a large surface area
A common way in which cells increase their surface area is division: when a cell splits itself in half, the cell’s internal volume remains the same, but the surface area is doubled. The cell can therefore take in nutrients twice as efficiently as before (2).
The surface area of a cell can also be increased by changing the cell’s shape. Having a smooth, spheroid outer layer does not allow for a particularly large surface area. However, if the membrane contains small infoldings, or small bumps/spikes, the surface area is considerably increased (3).
Why leaves need surface area
Whereas cells derive energy through diffusion of nutrients, leaves are fed by beams of sunlight. The more light a leaf absorbs, the more it can photosynthesise and therefore the more food is produced for the plant to which the leaf is attached. A larger plant will thus need larger leaves in order to maintain a sufficient supply of food (4).
How leaves ensure a large surface area
The surface area of a leaf is helped by its shape. Leaves are normally wide, flat and long. This increases the amount of material exposed to direct sunlight, while minimising the leaf’s internal volume (5). Like cells, leaves are often quite rough, because having multiple points which are concave or convex again increases the area. The final method to increase the input of light from leaves is to simply have more of them (6). This is especially useful for plants which grow in confined spaces, and thus lack sufficient room for large leaves, or whose stems are not strong enough to support them.
-
Chemical Symbols

A brief description of how three elements acquired their symbols.
Lead
The periodic symbol for lead is Pb. This is derived from the Latin word plumbum, meaning “soft metal”. Lead would have been known as plumbum nigrum, literally “soft black metal”. The Romans normally used lead for pipes (hence “plumber” and “plumbing”).
Gold
Gold has the symbol Au. This is also based on Latin: in the first and second centuries (C.E.), the Roman miners dislodged gold deposits from surrounding rock by blasting it with high-pressure jets of water. The Latin name for Gold was aurum.
Potassium
The symbol of potassium is K. There is no Latin term for potassium (as it was not differentiated from sodium until 1702), but the K symbol is based on the Neo-Latin word kalium, thought to mean “alkali”.